Hydrotalcite-supported palladium nanoparticles as catalysts for the hydroarylation of carbon–carbon multiple bonds†
Abstract
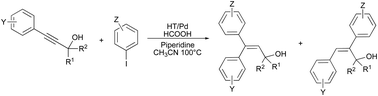
Palladium nanoparticles supported on Mg/Ca hydrotalcites catalyze the hydroarylation reaction of different alkynes and alkenes with aryl iodides under air in MeCN. The reaction of tertiary propargylic alcohols (1) with aryl iodides (2) yields, stereoselectively, γ,γ-diarylallylic alcohols (3) in moderate to high yields and high selectivity. Also, the HT/Pd hydroarylation reaction with aryl iodides was attempted on norbornene and α,β-unsaturated ketones affording, respectively, exo-aryl bicyclo[2.2.1]heptanes and β-aryl ketones in moderate to high yields. All the reactions described benefit from using a heterogeneous catalyst with evident advantages in terms of reaction purification and recyclability of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...