Efficient one-pot synthesis of enantiomerically pure N-protected-α-substituted piperazines from readily available α-amino acids†
Abstract
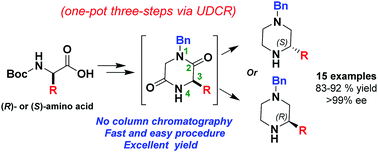
A new pathway towards enantiomerically pure 3-substituted piperazines, bearing a benzyl protecting group, has been developed in good overall yields (83–92%), starting from commercially available N-protected amino acids. The methodology represents an efficient and simple one-pot procedure, employing a synthetic sequence consisting of an Ugi-4 component reaction, a Boc-deprotection, an intramolecular cyclisation reaction and a final reduction (UDCR). From the benzyl protected precursors, the 2-substituted piperazines bearing a Boc-protecting group could consequently also be obtained via a simple protection and deprotection step of the corresponding piperazines. The practical utility of this methodology was demonstrated for chiral drug synthesis.


 Please wait while we load your content...
Please wait while we load your content...