Oxidation of p-toluic acid to terephthalic acid via a bromine-free process using nano manganese and manganese–copper mixed oxides
Abstract
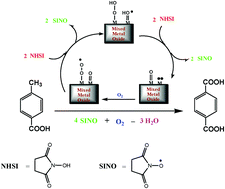
The industrial process of terephthalic acid (TPA) production suffers from many environmental and economical drawbacks. Liquid-phase catalytic oxidation of alkylbenzene by oxygen is usually initiated by bromine in a corrosive acetic acid solvent. A recent catalytic system used environmentally benign n-hydroxysuccinimide (NHSI) as a free radical promoter rather than the more hazardous bromine molecules. Manganese dioxide and Cu–Mn mixed oxide nanoparticles with different ratios (CuMn11, CuMn12, and CuMn13) were prepared and examined as oxidation catalysts for p-toluic acid (p-TA) to produce TPA under an oxygen atmosphere. These catalyst samples were characterized by HRTEM, XRD, and N2-adsorption–desorption isotherm techniques. The catalytic activity of the prepared nanocatalysts for TPA was studied under different conditions (reaction time, catalyst dosages, reaction solvent, and various oxygen pressures and reaction temperatures) to maximize the yield (%) and selectivity (%) towards TPA synthesis. Catalyst characterization indicated that the CuMn11 sample had a new phase with a spinel structure. Furthermore, it was the most active catalyst that provided 99.5% and 99.6% yields in acetonitrile and ethylacetate solvents, respectively, under the oxygen pressure of 20 atm. at 150 °C.


 Please wait while we load your content...
Please wait while we load your content...