Serine- and threonine-derived diamine equivalents for site-specific incorporation of platinum centers in peptides, and the anticancer potential of these conjugates†
Abstract
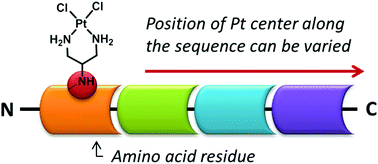
A modular strategy that allows introduction of one or more reactive platinum units at chosen locations along a peptide sequence is presented. This makes use of diazides generated from serine and threonine as diamine equivalents which can be conjugated to the peptide under standard coupling conditions. Reduction of these diazides using Pd/C and H2 followed by platination affords the final products in good yields. Following this, we prepared a new class of peptide–platinum conjugates and carried out preliminary cytotoxicity evaluation and DNA interaction studies. Inclusion of lysine residues in the sequence was found to improve DNA interaction and anticancer activities compared to analogous conjugates with hydrophobic side chains.


 Please wait while we load your content...
Please wait while we load your content...