Tetracarboxylic acids on a thiacalixarene scaffold: synthesis and binding of dopamine hydrochloride†
Abstract
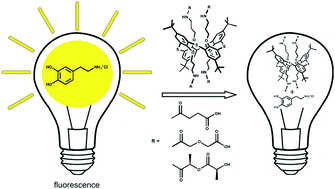
For the first time thiacalix[4]arene derivatives in 1,3-alternate conformation simultaneously containing amide, carboxyl and hydroxyl groups capable of forming 1 : 1 stoichiometry complexes with dopamine hydrochloride were obtained. The efficiency of dopamine hydrochloride binding was evaluated by a number of spectral methods. Using the methods of fluorescent, UV-Vis and NMR spectroscopy, the mechanism of interaction of the synthesized macrocycles with dopamine has been studied. It was shown that quenching of dopamine fluorescence by the studied macrocycles is carried out through a static mechanism.


 Please wait while we load your content...
Please wait while we load your content...