Looking at new ligands for chelation therapy†
Abstract
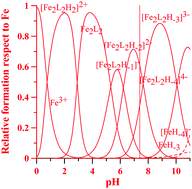
This work reports the synthesis, characterization and study of the complex formation equilibria of four new bis-kojic acid chelators with Fe3+, Al3+, Cu2+ and Zn2+. Based on previous encouraging results with tetradentate kojic acid derivatives, these ligands were designed with the aim of evaluating how acidic groups in the linker can affect both protonation constants and Fe3+ coordinating properties. Fe3+ and Al3+ complexation gave evidence of high metal-sequestering capacity, above all with the first metal ion. Complex formation equilibria with the essential metal ions Cu2+ and Zn2+ were furthermore studied to evaluate any disturbance of these chelating agents on the homeostatic equilibria of the essential metal ions. A multiplicity of techniques – potentiometry, UV-visible spectrophotometry, NMR spectroscopy and ESI-MS (electrospray ionization-mass spectrometry) – have enabled the characterization of the ligands, their corresponding metal complexes, together with an exhaustive analysis of the protonation and complex equilibria.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...