Synthesis and characterization of fused imidazole heterocyclic selenoesters and their application for chemical detoxification of HgCl2†
Abstract
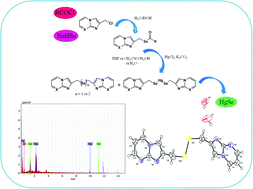
A new series of selenoester derivatives of imidazo[1,2-a]pyridine and imidazo[1,2-a]pyrimidine was synthesized under mild conditions using a simple methodology by the reaction of in situ generated sodium selenocarboxylates with 2-(chloromethyl)imidazo[1,2-a]pyridine/pyrimidine (3a–b) in water and ethanol. Excellent yields of phenyl/4-tolyl selenoesters of imidazo[1,2-a]pyridine/pyrimidine (6a–b and 6e–f) were obtained in water, whereas 4-methoxyphenyl/4-chlorophenyl/2-thienyl selenoesters of imidazo[1,2-a]pyridine/pyrimidine (6c–d and 6g–i) could only be synthesized in ethanol. Further, a series of reactions was carried out with 6e as a model compound in order to study the reaction behavior of selenoesters with mercury(II) chloride (HgCl2). The deprotection of compound 6e by HgCl2 was carried out in the presence of K2CO3 under different conditions to obtain bis(imidazo[1,2-a]pyrimidin-2-ylmethyl)selenide (8) and bis(imidazo[1,2-a]pyrimidin-2-ylmethyl) diselenide (9) along with a small amount of less soluble bis((imidazo[1,2-a]pyrimidin-2-ylmethyl)selanyl)mercury (10). The mercury–selenium complex 10 has a short shelf life and decomposes to give HgSe, which was characterized using EDX analysis. The structure of compound 9 was established with X-ray crystallography. The formation of HgSe indicates that the selenoester derivatives may prove useful in the treatment of HgCl2 induced toxicity.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...