Proton triggered emission and selective sensing of 2,4,6-trinitrophenol using a fluorescent hydrosol of 2-phenylquinoline†
Abstract
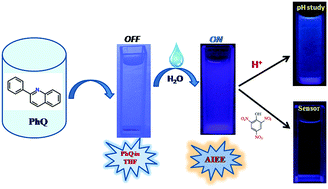
2-Phenylquinoline (PhQ) displayed novel aggregation induced emission enhancement (AIEE) characteristics in its aggregate/solid state. It also allows reversible fluorescence switching in acidic and basic media and changes the emission color from sky blue to intense blue in its aggregate state through protonation. Such behavior enables it to be utilized as a fluorescent pH sensor in acidic and basic media. PhQ microparticles with distinct morphologies are synthesized through a reprecipitation method and SDS is used as the morphology directing agent. The photophysical properties, size and growth process of the particles are characterized by UV-Vis absorption, steady state and time resolved spectroscopy, optical and scanning electron microscopy studies. The “turn off” luminescence property of the aggregated PhQ hydrosol in the presence of 2,4,6-trinitrophenol (TNP) is used for the selective detection of trace amounts of TNP in water and the super-amplified fluorescence quenching has been explained as due to ground state complexation between PhQ and TNP.


 Please wait while we load your content...
Please wait while we load your content...