Regioselective addition of DDQ on a quinoid ring: an entry into chiral zwitterionic bridging ligands†
Abstract
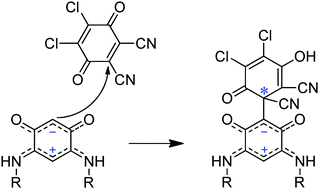
The regioselective insertion of DDQ into a C–H bond of the 6π + 6π electron zwitterionic benzoquinonemonoimines 4a–c results in the formation of novel chiral C-substituted quinoid ligands 11a–c. These Michael adducts feature a preserved zwitterionic form and a quaternary stereogenic carbon center as evidenced by the single crystal X-ray structure of the derivative 11a. The ECD spectra, optical rotations and racemization barriers of the two enantiomers of 11a were measured subsequently to their separation using preparative chiral HPLC. Because the racemization of 11a is stopped in basic media, compounds 11a–c give a new entry in chiral zwitterionic bridging ligands.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...