Preparation of metal oxide supported catalysts and their utilization for understanding the effect of a support on the catalytic activity†
Abstract
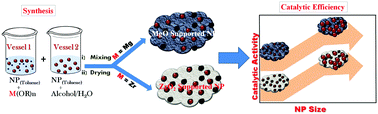
A convenient way of anchoring transition metal nanoparticles (palladium, platinum, rhodium and ruthenium) onto metal oxide supports (magnesium oxide and zirconium oxide) by means of a modified sol–gel technique is demonstrated. Use of toluene dispersed, ligand protected pre-synthesized nanoparticles during sol–gel synthesis delivered size-controlled, spatially distributed, well-adhered transition metal nanoparticles (MNPs) on metal oxide supports. The catalytic activities of these supported nanoparticles were tested for the p-nitro phenol reduction reaction. It was observed that the reaction kinetics were crucially dependent on the catalyst support and MNP size. The influence of the magnesium oxide and zirconium oxide supports towards the catalytic performance of the anchored transition MNPs was probed using cyclic voltammetry and the differences in the same were attributed to the support-induced modification in the electronic properties of the MNPs. Our results indicated that magnesium oxide is a better support than zirconium oxide.


 Please wait while we load your content...
Please wait while we load your content...