Understanding the structure and reactivity of the C–S linkage in biologically active 5-arylthio-5H-chromenopyridines†
Abstract
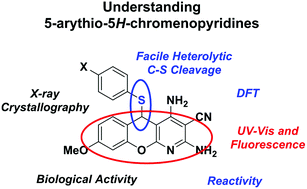
Molecules based on the 5-arylthio-5H-chromenopyridine structure have been studied for a variety of medicinal applications, including liver fibrosis. We have recently found that the C–S linkage can be cleaved with Pt(II) and Au(I), something that was not previously recognized. In this current work, we examine the structure, properties, and reactivity of 5-arylthio-5H-chromenopyridines using spectroscopy, crystallography, reactivity, density functional theory (DFT) calculations, and electrochemistry. We report the crystal structures of 5-arylthio-5H-chromenopyridines, as well as their DFT-calculated structures and electronic structures. The DFT calculations help to explain the electronic properties of these complexes, such as the observation that “soft” metals cleave the C–S bond. We find that the arylthio group is a good leaving group on these molecules due to the stability of the carbocation that is formed, and can be removed via reduction with NaBH4. Methoxy-substituted chromenopyridines were found to be viable at inhibiting liver fibrosis. These results will help inform the biological studies with these compounds to determine method of action and enhance development of new drug molecules with increased stability and activity based on the 5H-chromenopyridine scaffold.


 Please wait while we load your content...
Please wait while we load your content...