Lanthanide oxalatophosphonates with two types of layered structures: syntheses, structures, luminescence and magnetic properties†
Abstract
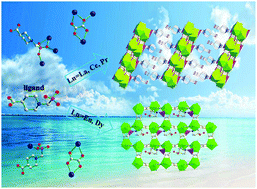
Two types of lanthanide(III) oxalatophosphonates, namely, [Ln(H2L)(C2O4)]·H2O [Ln = La (1), Ce (2), Pr (3)] and [Ln2(H3L)(C2O4)3(H2O)4]·2H2O [Ln = Eu (4) and Dy (5)] have been hydrothermally obtained by using (4-carboxypiperidyl)-N-methylenephosphonic acid (H3L) as the phosphonate ligand and oxalate as the second ligand. These five compounds have the same precursor, but they exhibit two different 2D layered structures. Compounds 1–3 are isostructural and exhibit a 2D layered structure in which {Ln(1)O9}, {Ln(2)O9} and {CPO3} polyhedra via corner-sharing and edge-sharing are interconnected into a 1D chain. These chains are further cross-linked by oxalate anions into a 2D layered structure, and the uncoordinated carboxylate groups are oriented toward the interlayer space. Compounds 4 and 5 are isostructural and also show a 2D layered structure. Lanthanide ions are interconnected by oxalate anions forming a 2D layered structure, and the carboxyl groups of H3L are grafted on the two sides of the layer. The structure variations are attributed to the effect of lanthanide contraction. In addition, the luminescence properties of compounds 4 and 5, and the magnetic properties of compound 5 have been studied.


 Please wait while we load your content...
Please wait while we load your content...