Hexanuclear iron(iii) α-aminophosphonate: synthesis, structure, and magnetic properties of a molecular wheel†
Abstract
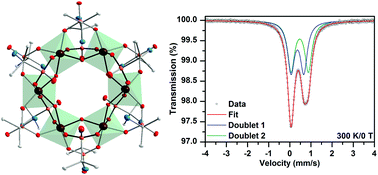
A new hexanuclear molecular iron phosphonate complex, [Fe6(HAIPA)12(OH)6]·nH2O (1·nH2O) (H2AIPA = NH2(CH3)2CP(O)(OH)2, (2-aminopropan-2-yl)phosphonic acid), was synthesized from Fe2+ and Fe3+ salts in water by interaction with the ligand salts. Addition of corresponding amounts of sodium or tetramethylammonium salts of H2AIPA to the solution of iron precursors led to the formation of large bright-green crystals of complex 1. Isolated products were studied by spectroscopic and analytical methods – IR, Mössbauer spectroscopy, TG/DSC, ICP-OES, and CHN analysis. A novel {Fe6} hexanuclear molecular structure of 1 was confirmed by single crystal X-ray diffraction analysis. An octahedral coordination environment of iron cations is formed by phosphonate and hydroxo oxygens. Twelve phosphonate groups and six –OH groups act as bridging ligands and bind six Fe octahedra. Because of protonation of the amino group, the phosphonate anions coordinate in the zwitterionic form as HAIPA− (NH3+(CH3)2CPO32−). The iron cations are present in the form of high-spin Fe3+, which was confirmed by the bond valence sum (BVS) calculations and the 57Fe Mössbauer spectra. The magnetic measurements show antiferromagnetic coupling between the iron centers with decreasing temperature.


 Please wait while we load your content...
Please wait while we load your content...