Effect of BaCO3 addition on the CO2-derived carbon deposition in molten carbonates electrolyzer
Abstract
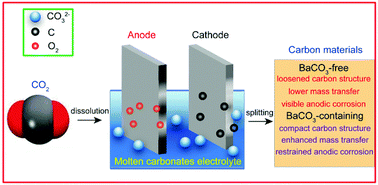
Electrolysis of molten carbonates provides an effective means to achieve the direct conversion of the greenhouse gas carbon dioxide to readily available carbonaceous fuel or chemicals. In this study, CO2 was electrolyzed in a Li–Na–K ternary carbonates mixture using galvanized Fe as the cathode and nickel as the anode. In particular, the effect of BaCO3 on the generation of carbon was studied. The results demonstrate that BaCO3 addition facilitates the deposition of compact carbon materials. Moreover, decreasing the temperature and increasing the current density lead to the formation of loosened carbon products. Though Li–Na–K–Ba electrolysis exhibits a higher cell voltage than that using Li–Na–K carbonates, BaCO3 addition can significantly ease anode corrosion and the corresponding corrosion inhibiting action is discussed. In summary, a facile CO2 absorption and a mitigatory nickel anode corrosion were observed when BaCO3 was dissolved in Li–Na–K carbonates and further investigation is expected.


 Please wait while we load your content...
Please wait while we load your content...