Theoretical and experimental characterization of 1,4-N⋯S σ-hole intramolecular interactions in bioactive N-acylhydrazone derivatives†
Abstract
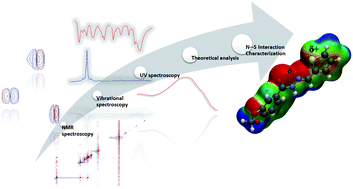
Sigma-hole (σ-hole) bonds are interactions that are gaining special attention in medicinal chemistry. This type of interaction, initially assigned to the halogens (group 17 of the periodic table), has been extended to atoms of groups 14, 15 and 16. Sulfur atoms have been outstanding for describing these interactions at the intramolecular level (to induce conformational stability) and the intermolecular level (participating in molecular recognition of bioactive compounds by their respective targets). Thus, this work describes the theoretical and experimental characterization of a 1,4-N⋯S σ-hole intramolecular interaction in the N-acylhydrazone cardioactive prototype LASSBio-294 (1), which leads to conformational stabilization and has a direct influence on the molecular properties of this inotropic prototype compared to a negative control for the interaction, LASSBio-897 (2), which is the regioisomer at the thiophene ring. Our theoretical results were reached using the B3LYP/6-311G(d) level of theory, including analysis of conformational, orbital and electrostatic properties. We performed experimental studies using IR, Raman, UV and NMR spectroscopies, which corroborated our theoretical data, showing significant differences between LASSBio-294 (1) and LASSBio-897 (2) in relation to the bond strength of the groups involved in the N⋯S interaction (S–C and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds), the energies of the orbitals associated with the S lone pair (Lp(S)) and the antibonding N
C bonds), the energies of the orbitals associated with the S lone pair (Lp(S)) and the antibonding N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C π bond (π*(N
C π bond (π*(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)), as well as the 15N chemical shifts in both systems. Together, our results show how this unusual interaction can influence the molecular properties of some organic compounds.
C)), as well as the 15N chemical shifts in both systems. Together, our results show how this unusual interaction can influence the molecular properties of some organic compounds.


 Please wait while we load your content...
Please wait while we load your content...