Structural insights into a novel anticancer sulfonamide chalcone†
Abstract
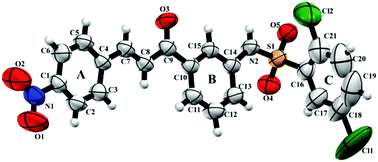
Natural products have stood out due to their wide range of biological activities. Among the various naturally occurring classes, we can highlight chalcones, sulfonamides and hybrid compounds formed by both. Although many pharmacological activities of these classes of compounds are known, new ones have arisen lately and require detailed structural and biological analyses. Herein, we report the synthesis and structural elucidation of a novel sulfonamide chalcone 2,5-dichloro-N-{3-[(2E)-3-(4-nitrophenyl)prop-2-enoyl]phenyl}benzenesulfonamide (BSC) by Single Crystal X-ray Diffraction and spectroscopy analysis (Infrared, NMR and Mass Spectroscopy). Topology was determined through Hirshfeld surface analysis and the electrostatic potential map, while the energy of frontier molecular orbitals was used to evaluate the stability of BSC. Additionally, the cytotoxicity of the title compound was evaluated through the MTT colorimetric method. We show that the BSC compound has a planar conformation in its chalcone portion, which is further corroborated by the low angle between the aromatic rings (5.23°). In addition, intermolecular interactions of type C–H⋯O and N–H⋯O make up a dimeric supramolecular arrangement. An inverse virtual screening approach allowed identifying potential biological applications for BSC, which indicated that BSC might interact with the binding sites of RARα and RARβ. Consequently, BSC was experimentally evaluated against three cancer cell lines, and was shown to hold potent anticancer activity. In addition, a cytotoxic effect was observed for all strains, which was more pronounced for HCT-116, a colon cancer cell line.


 Please wait while we load your content...
Please wait while we load your content...