Diketopyrrolopyrrole based organic semiconductors with different numbers of thiophene units: symmetry tuning effect on electronic devices†
Abstract
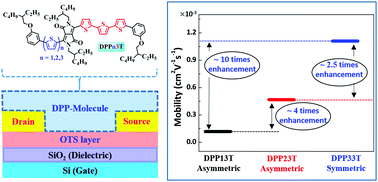
Diketopyrrolopyrrole (DPP) has been drawing considerable attention for constructing semiconducting materials used in organic optoelectronic applications, mainly for organic field effect transistor (OFET) and organic photovoltaic (OPV) devices. In the present work, we study the effects of varying the number of thiophene units (from four to six) attached to DPP on the physical, chemical, and optoelectronic properties by designing and synthesizing a series of small molecule organic semiconductors. The thermal and optical properties, and electronic energy levels of these molecular semiconductors are studied, and their performance in organic field effect transistor devices (OFETs) compared. These small molecules exhibit promising charge carrier mobility and behave as p-type semiconductors. Hole mobility increases with conjugation length and degree of symmetry of the backbone. By adjusting the number of thiophene units on each side of DPP, the hole mobility is enhanced by almost one order of magnitude, from 1.18 × 10−4 to 1.11 × 10−3 cm2 V−1 s−1. Density functional theory (DFT) calculations indicate that increasing the number of homo-coupled thiophene results in a relatively planar configuration, while the terminal alkoxyl benzene unit causes significant torsional rotation which could hamper electron and hole transport in active layer.


 Please wait while we load your content...
Please wait while we load your content...