Novel polymerization of aniline and pyrrole by carbon dots
Abstract
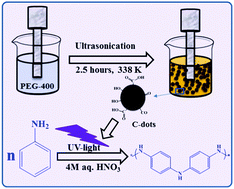
The polymerization of aniline requires an initiator, such as ammonium persulfate, iron chloride (FeCl3), potassium persulfate, or silver nitrate. In this study, we introduced a novel one-pot synthesis of polyaniline using carbon dots (C-dots) and UV light that act as catalysts without any additional initiator. Chemical oxidative polymerization is a facile one-step process where C-dots are prepared from polyethylene glycol-400 (PEG-400). Polyaniline was also synthesized by employing carbon dots coated on a glass plate and irradiation of UV light. The synthesized polymer was characterized using Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), thermogravimetric analysis (TGA), scanning electron microscopy (SEM) and solid-state NMR spectroscopy. A similar procedure also allowed the synthesis of polypyrrole from pyrrole. The approach using only carbon dots and UV light offers a novel and facile route towards the formation of polymers.


 Please wait while we load your content...
Please wait while we load your content...