Unravelling 2-aminoquinazolin-4(3H)-one as an organocatalyst for the chemoselective reduction of nitroarenes†
Abstract
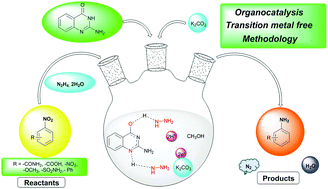
A novel, mild and transition metal-free, 2-aminoquinazolin-4(3H)-one-assisted reduction of nitroarenes employing hydrazine hydrate as reducing agent and potassium carbonate as a base is reported. For the first time, the activation of hydrazine hydrate with an organocatalyst has been explored for reduction reactions. Also for the first time, 2-aminoquinazolin-4(3H)-one and its derivatives have been investigated as hydrogen bonding organocatalysts for the reduction of nitroarenes to anilines. Sensitive functional groups such as sulfonamide, carboxyl, amide and halides were well tolerated in this green methodology with scalability and high chemoselectivity.


 Please wait while we load your content...
Please wait while we load your content...