A uniquely fabricated Cu(ii)-metallacycle as a reusable highly sensitive dual-channel and practically functional metalloreceptor for Fe3+ and Ca2+ ions: an inorganic site of cation detection†
Abstract
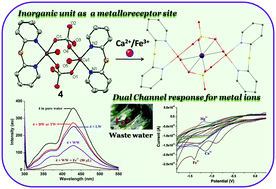
The present study deals with the formation of cyanuric chloride·1,2-di(pyridin-2-yl)disulfane 1 co-crystal and trimethylcyanuric acid 2 through unusual reactions of cyanuric chloride (CC). Reaction of co-crystal 1 with Co(II) and Cu(II) led to the formation of di(pyridin-2-yl)sulfane 1′ through a desulfurization reaction and complexes [Co(1′)Cl2] (3) and [Cu(1′)2-(μ2-SO4)2(H2O)2] (4). The structures of 1–4 were verified using X-ray single crystal analysis. The Cu(II)-metallacycle 4 acts as a dual-channel metalloreceptor for Fe3+ and Ca2+ through a fluorescence ‘turn-on’ response for Ca2+, a ‘turn-off’ response for Fe3+, and changes in cyclic voltammograms in pure aqueous-buffer medium (1.0 M Tris–HCl, pH 7.4). The limits of detection (LOD) for Fe3+ and Ca2+ were estimated to be 30 ppb and 0.2 ppm, respectively. For the first time, the EPR technique was applied to detect a paramagnetic ion (Fe3+) using a paramagnetic probe (4), which exhibited considerable change upon interaction with Fe3+. Further, structural elucidation of 4, use of Co(II)-complex 3 as a model compound, and FTIR and mass spectral studies suggested that the detection of cations by Cu(II)-metallacycle 4 occurs through its unique inorganic detection site (six oxygen atoms from sulphate and water groups). Due to its higher selectivity of Fe3+ over Ca2+ and other metal ions as well as its LOD for Fe3+, which is lower than the US Environmental Protection Agency permitted maximum level in drinking water (5.4 μmol L−1), 4 was successfully used for practical detection of Fe3+ in environmental water samples. The exceptionality of this report comprises the detection of vital cations using a binuclear Cu(II) metallacycle probe, utilizing the EPR technique, binding through an inorganic site and the practical application of 4 toward Fe3+ sensing.


 Please wait while we load your content...
Please wait while we load your content...