Fluorescent β-ketothiolester boron complex: substitution based “turn-off” or “ratiometric” sensor for diamine†
Abstract
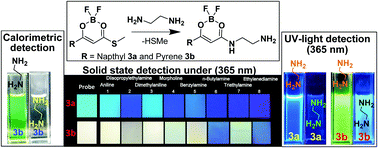
Fluorophore substituted boron diketonate 3 shows solvatochromism with a large Stokes shift ranging from 121 nm to 166 nm and emitting strongly in its solid states 3a (463 nm) and 3b (563 nm). Boron diketonate provides a choice of fast turn-off or ratiometric detection of a diamine with respect to the fluorophore substitution on the boron-chelating ring. Naphthyl substituted 3a emission was quenched rapidly and selectively on interaction with ethylenediamine. The detection limit was found to be 0.8 μM. Pyrene substituted 3b exhibited selective calorimetric and ratiometric detection of ethylenediamine with a detection limit of 1.2 μM. Diamine involves a substitution reaction with the elimination of methylsulfanyl group in 3 and it was supported by NMR and mass techniques. The rates of reaction of 3a and 3b with ethylenediamine, Kobs, were determined and found to be 2.18–9.96 × 10−4 s−1 in chloroform. Detection of vaporized diamine over other monoamines under UV-light (365 nm) was achieved with a demonstration of boron diketonate 3 coated silica gel TLC plate test strip array.


 Please wait while we load your content...
Please wait while we load your content...