The molecular design of cage metal complexes for biological applications: pathways of the synthesis, and X-ray structures of a series of new N2-, S2- and O2-alicyclic iron(ii) di- and tetrachloroclathrochelates†
Abstract
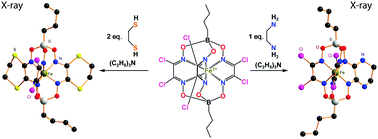
The synthesis of new metal(II) di- and tetrahalogenoclathrochelates with apical functionalizing substituents as reactive macrobicyclic precursors is a key stage of the molecular design of cage metal complexes – prospective biological effectors. We found that the most convenient multistep synthetic pathway for their preparation includes (i) direct template condensation of a dihalogeno-α-dioxime with an appropriately functionalized boronic acid on the corresponding metal ion as a matrix, giving an apically functionalized metal hexahalogenoclathrochelate in a high yield; and (ii) its stepwise nucleophilic substitution with S2-, N2- or O2-bis-nucleophiles, forming stable six-membered alicyclic ribbed fragments, thus allowing obtaining the corresponding apically functionalized di- and tetrahalogenoclathrochelates. The latter reaction of an iron(II) hexachloroclathrochelate with different N2-, S2- and O2-bis-nucleophilic agents afforded chloroclathrochelate complexes with equivalent and non-equivalent alicyclic ribbed substituents, such as N2-, S2 or O2-containing six-membered cycles. In the case of anionic forms of pyrocatechol and 1,2-ethanedithiol as O2- and S2-bis-nucleophiles, generated in situ in the presence of triethylamine, such substitution proceeds easily and in a high yield. In the case of anionic derivatives of ethylenediamine as N2-bis-nucleophiles, only a mono-N2-alicyclic iron(II) tetrachloroclathrochelate was obtained in a moderate yield. The S2-alicyclic iron(II) tetrachloroclathrochelate underwent a further nucleophilic substitution of one of the two dichloroglyoximate fragments, giving its N2, S2-alicyclic dichloroclathrochelate derivative with three non-equivalent ribbed chelate fragments. The complexes obtained were characterized using elemental analysis, MALDI-TOF mass spectrometry, and IR, UV-vis, 1H and 13C{1H} NMR spectroscopies, and by single crystal X-ray diffraction (XRD). As follows from XRD data for four O2-, S2- and N2-ribbed-functionalized iron(II) clathrochelates, the geometry of their FeN6-coordination polyhedra is intermediate between a trigonal prism and a trigonal antiprism. UV-vis spectra of these cage complexes are indicative of a dramatic redistribution of the electron density in a quasiaromatic clathrochelate framework caused by its ribbed functionalization with six-membered O2-, S2- and/or N2-alicyclic substituent(s).


 Please wait while we load your content...
Please wait while we load your content...