X-ray crystal structures and anti-breast cancer property of 3-tert-butoxycarbonyl-2-arylthiazolidine-4-carboxylic acids†
Abstract
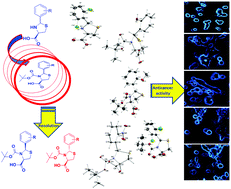
Diastereomeric ‘2RS,4R’-2-arylthiazolidine-4-carboxylic acids (ATCAs) were synthesized and their resolution to chiraly pure N-BOC derivatives was attempted by column chromatography. The absolute stereochemistry of the resolved compounds was ascertained by X-ray single crystal structures. Further application of the synthesized compounds was studied for their in vitro anti-breast cancer activity against MCF7 cell line using DOX as a standard by MTT assay method. Cell morphology analysis was carried out by fluorescence microscopy. The compounds containing ‘2S’ absolute configuration in thiazolidine ring and presence of 2-NO2, 2,6-Cl groups on ‘2R’-aryl substituent showed significant anti-breast cancer activity where some of the compounds were found to be more active than DOX in terms of induced apoptosis mode of MCF7 cell death.


 Please wait while we load your content...
Please wait while we load your content...