Light-assisted preparation of a cyclodextrin-based chiral stationary phase and its separation performance in liquid chromatography†
Abstract
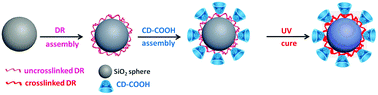
A cyclodextrin-based chiral stationary phase (CD-CSP) is one of the most widely applied CSPs due to its powerful enantioseparation ability. In this study, a facile method was developed to prepare a CD-CSP via carboxyl methyl β-cyclodextrin (CD-COOH) and diazo-resin (DR). Monodisperse silica particles were synthesized using a modified Stöber method. Then DR and CD-COOH were coated on the silica particles via ionic bonding successively and UV light was finally used to couple silica, DR and CD-COOH and the ionic bonds turned into covalent bonds. The resultant CD–DR silica particles were characterized using Fourier transform infrared spectroscopy (FT-IR), thermo-gravimetric analysis (TGA) and scanning electron microscopy (SEM). The enantioselectivity of the CD@SiO2 particles was explored in reversed phase high-performance liquid chromatography (RP-HPLC). Baseline separation of chiral drugs was achieved and the effects of separation parameters (elution mode, buffer and analyte mass) were investigated in detail. By using water soluble non-toxic DR to replace a highly toxic and moisture sensitive silane agent to modify silica microspheres, this light-assisted strategy can provide a green and effective technique to manufacture packing materials for enantioseparation applications.


 Please wait while we load your content...
Please wait while we load your content...