Copper-catalyzed synthesis of benzanilides from lignin model substrates 2-phenoxyacetophenones under an air atmosphere
Abstract
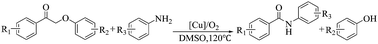
The synthesis of chemicals from biomass-derived compounds is an interesting and challenging topic. In this work, using the lignin-derived 2-phenoxyacetophenones as the feedstock we present a novel approach for the synthesis of benzanilides via the reaction of 2-phenoxyacetophenones with anilines catalyzed by CuCl2 in DMSO at 120 °C under an air atmosphere. This approach has wide scope for 2-phenoxyacetophenones and anilines, and various benzanilides accompanied by the corresponding phenols could be obtained in high yields via changing the 2-phenoxyacetophenones and anilines. The reaction mechanism study indicated that the oxidative cleavage of the C–C bond in 2-phenoxyacetophenones and the formation of a C–N bond occurred simultaneously in the reaction process, resulting in the formation of benzanilides together with phenols.


 Please wait while we load your content...
Please wait while we load your content...