Fe3O4/PEG-SO3H as a heterogeneous and magnetically-recyclable nanocatalyst for the oxidation of sulfides to sulfones or sulfoxides
Abstract
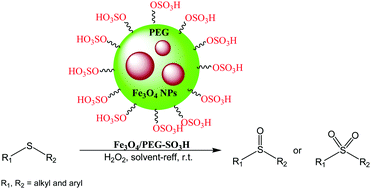
We present below a sulfonated-polyethylene glycol-coated Fe3O4 nanocomposite (Fe3O4/PEG-SO3H) as a greatly effective and ecological nanocatalyst for the selective oxidation of sulfides to sulfoxides or sulfones with brilliant yields under solvent-free conditions by employing 30% hydrogen peroxide as the oxidant. A number of sulfides containing alcohol, ester, and aldehyde functional groups were fruitfully and selectively oxidized without altering the desired characteristics. The magnetic nanocatalyst (Fe3O4/PEG-SO3H) can be conveniently and swiftly retrieved through the utilization of an external magnetic tool and recycled for more than 10 reaction runs without significantly decreasing its catalytic behavior.


 Please wait while we load your content...
Please wait while we load your content...