Biochemical and biophysical insights into the metal binding spectrum and bioactivity of arginase of Entamoeba histolytica†
Abstract
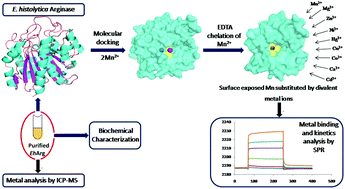
The human protozoan pathogens possess the essential metalloenzyme arginase (Arg) which catalyses the catabolism of L-arginine to L-ornithine and urea. This being the first committed step in polyamine biosynthesis is a potential drug target for protozoan diseases. In pathogenic organisms, arginase plays a crucial role in depleting host L-arginine, a substrate for nitric oxide synthase (NOS) that participates in protective immunity, thereby evading host immune response. In this study, the metal binding spectrum of EhArg has been determined. This study focuses on the biochemical and biophysical characterization of arginase from Entamoeba histolytica (EhArg), majorly characterizing the bivalent metal selectivity and metal binding kinetics of purified EhArg using Surface Plasmon Resonance and inductively coupled plasma mass spectroscopy. Investigation of the active site chemistry and total metal content using molecular docking and ICP-MS unraveled the fact that two Mn2+ ions are required for the enzyme to be fully functional. However, chelating loosely bound Mn2+ and replacing it with a variety of bivalent metal ions including Mg2+, Zn2+, Ni2+, Hg2+, Cu2+, Co2+, Ca2+ and Cd2+ retains its enzymatic activity. Further, the role of nine bivalent ions in the activation of EhArg was studied thermodynamically and biochemically. Phylogenetic and sequence analysis and oligomerization studies of EhArg show that unlike other eukaryotic arginases, EhArg exists in monomeric and dimeric form in solution and shows the highest similarity with bacterial arginase. This study unveiled interesting facts about EhArg that the enzyme has evolved to utilize available metal ion cofactors and survive the inhospitable environment within the host.


 Please wait while we load your content...
Please wait while we load your content...