Positive and negative nano-electrospray mass spectrometry of ruthenated serum albumin supported by docking studies: an integrated approach towards defining metallodrug binding sites on proteins†
Abstract
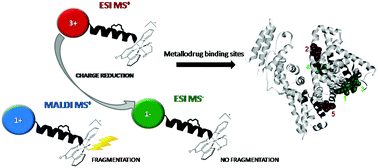
Binding of three ruthenium(II) compounds of general formula mer-[Ru(L3)(N-N)X][Y] (where L3 = 4′-chloro-2,2′:6′,2′′-terpyridine (Cl-tpy); N-N = 1,2-diaminoethane (en), 1,2-diaminocyclohexane (dach) or 2,2′-bipyridine (bipy); X = Cl; Y = Cl) to human serum albumin (HSA) has been investigated by nano-LC/nano-ESI MS and docking studies. A bottom-up proteomics approach has been applied for the structural characterization of metallated proteins and the data were analyzed in both the positive and negative ion mode. The negative ion mode was achieved after the post-column addition of an isopropanol solution of formaldehyde that enabled sample ionization at micro-flow rates. The negative ion mode MS has been proved to be beneficial for the analysis of binding sites on ruthenated protein in terms of ion charge reduction and consequent simplification of target sequence identification based on isotopic differences between ruthenated and non-ruthenated peptides. Moreover, the negative ion mode ESI MS shows the advantage of singly charged ion formation and, unlike MALDI MS, it does not cause complete ligand fragmentation, merging the benefits of each method into a single experiment. Six target sequences were identified for the binding of en and dach compounds, and four sequences for the binding of bipy. All compounds have been found to bind histidine and one aspartate residue. Docking studies showed that the identified sequences are the constituents of five distinct binding sites for en and dach, or two sites for the bipy complex. The selection of binding sites seems to be dependent on the chelate ligand and the form of the complex prior or after hydrolysis of the leaving chloride ligand.


 Please wait while we load your content...
Please wait while we load your content...