Modulation of thiol-dependent redox system by metal ions via thioredoxin and glutaredoxin systems
Abstract
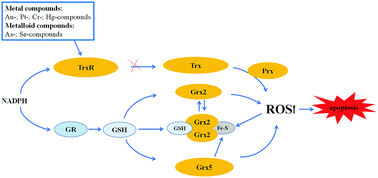
The thioredoxin and glutaredoxin systems possess a variety of biological activities in mammalian cells, including the defense against oxidative stress, regulation of DNA synthesis, the cell cycle and the mediation of apoptosis. The thioredoxin system, comprised of NADPH, thioredoxin reductase (TrxR) and thioredoxin (Trx), exerts its activities via a disulfide–dithiol exchange reaction. Mammalian TrxRs are selenoproteins; the thiols and selenols in the active site of these enzymes confer the thioredoxin system to work as soft bases, which have a high affinity with soft acids, including numerous metal ions. In this review we focus on recent advances in the modulation of thioredoxin and glutaredoxin systems by metal ion soft acids. Numerous clinical metal-containing drugs, such as platinum- and gold-containing compounds, show inhibitory effects on the thioredoxin system, providing strategies to develop novel anti-cancer drugs. Moreover, inhibition of the Trx system by soft acids, such as mercury-, chromium- and arsenic-containing compounds cause changes in the cellular redox state and contribute to their cell toxicity. In addition, metal ions are also involved in the regulation of the glutaredoxin system. Iron ions participate in regulating Grx2 activity via iron–sulfur cluster formation. Moreover, Grx5 in mitochondria contains a 2Fe–2S cluster stabilized by GSH, which can mediate cellular iron metabolism. Collectively, these results demonstrate that metal ions are major players in regulating the Trx and Grx systems-mediated cellular redox processes and thus, provide an opportunity to understand the functions of metal ions in thiol metabolism dysfunction-related diseases.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...