Removal of the Fe(iii) site promotes activation of the human cystic fibrosis transmembrane conductance regulator by high-affinity Zn(ii) binding†
Abstract
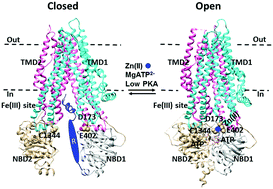
The cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel is activated by ATP binding at the interface of two cytoplasmic nucleotide binding domains (NBDs) and phosphorylation of the regulatory (R) domain by protein kinase A (PKA). The human CFTR has two functionally active thiol groups for gating regulation by chemical modification. Although modification of C832 in the R domain with N-ethylmaleimide promotes channel opening, glutathionylation of C1344 in NBD2 inhibits channel opening. Our recent studies demonstrated that the N-ethylmaleimide-induced potentiation involves a high-affinity inhibitory Fe3+ site at the interface between the R domain and intracellular loop 3 (ICL3). However, it is unknown whether the glutathionylation-evoked inhibition implies another stimulatory metal site. Here, Fe3+-insensitive mutations at the R–ICL3 interface were employed to further examine whether Zn2+ potentiated the activity of the human CFTR channel by targeting C1344 once the interfacial Fe3+ bridge was disrupted. The results showed that internal nanomolar Zn2+ increased its activity by about two- to threefold at a low level of protein kinase A, and the increase was reversed by EDTA or DTT or reduced glutathione but suppressed by a high level of protein kinase A, N-ethylmaleimide modification or a C1344A mutation. It is interesting that this Zn2+-triggered potentiation is not found in the wild type human CFTR to which endogenous Fe3+ is bound. Thus, the high-affinity binding of Zn2+ to C1344 in NBD2 may stimulate human CFTR activity in a phosphorylation-dependent manner, but the primary binding of Fe3+ to the ICL3–R interface may prohibit this stimulation.
- This article is part of the themed collection: Iron in Biology


 Please wait while we load your content...
Please wait while we load your content...