Evaluation of Cu(i) binding to the E2 domain of the amyloid precursor protein – a lesson in quantification of metal binding to proteins via ligand competition†
Abstract
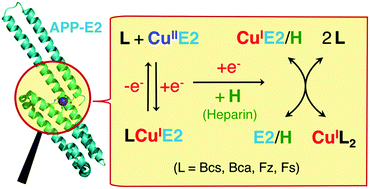
The extracellular domain E2 of the amyloid precursor protein (APP) features a His-rich metal-binding site (denoted as the M1 site). In conjunction with surrounding basic residues, the site participates in interactions with components of the extracellular matrix including heparins, a class of negatively charged polysaccharide molecules of varying length. This work studied the chemistry of Cu(I) binding to APP E2 with the probe ligands Bcs, Bca, Fz and Fs. APP E2 forms a stable Cu(I)-mediated ternary complex with each of these anionic ligands. The complex with Bca was selected for isolation and characterization and was demonstrated, by native ESI-MS analysis, to have the stoichiometry E2 : Cu(I) : Bca = 1 : 1 : 1. Formation of these ternary complexes is specific for the APP E2 domain and requires Cu(I) coordination to the M1 site. Mutation of the M1 site was consistent with the His ligands being part of the E2 ligand set. It is likely that interactions between the negatively charged probe ligands and a positively charged patch on the surface of APP E2 are one aspect of the generation of the stable ternary complexes. Their formation prevented meaningful quantification of the affinity of Cu(I) binding to the M1 site with these probe ligands. However, the ternary complexes are disrupted by heparin, allowing reliable determination of a picomolar Cu(I) affinity for the E2/heparin complex with the Fz or Bca probe ligands. This is the first documented example of the formation of stable ternary complexes between a Cu(I) binding protein and a probe ligand. The ready disruption of the complexes by heparin identified clear ‘tell-tale’ signs for diagnosis of ternary complex formation and allowed a systematic review of conditions and criteria for reliable determination of affinities for metal binding via ligand competition. This study also provides new insights into a potential correlation of APP functions regulated by copper binding and heparin interaction.


 Please wait while we load your content...
Please wait while we load your content...