The antioxidant 2,6-di-tert-butylphenol moiety attenuates the pro-oxidant properties of the auranofin analogue†
Abstract
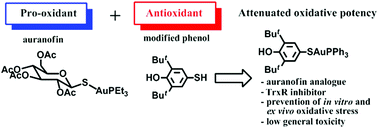
Metal-based drugs are gaining momentum as a rapidly developing area of medicinal inorganic chemistry. Among gold pharmaceuticals, auranofin is a well known antirheumatic drug. The efficacy of gold–organic complexes largely depends on their pro-oxidant properties since auranofin targets the redox enzyme thioredoxin reductase (TrxR). However, an uncontrollable oxygen burst may be harmful for healthy cells; therefore, the search for chemical modifications to attenuate oxidation-related general toxicity of gold containing anti-inflammatory drugs is justified. In this study, we demonstrate that the incorporation of a specific antioxidant phenol fragment can counterbalance the pro-oxidative potential of the Au containing complex molecule. The electrochemical studies of AuPPh3SR (1, R= 3,5-di-tert-butyl-4-hydroxyphenyl) and its precursors AuPPh3Cl (2) and RSH (3) showed that complex 1 and phenol 3 efficiently scavenged the radicals (as detected by cyclic voltammetry) whereas 2 had no effect. Compound 1 inhibited TrxR in vitro with IC50 0.57 ± 0.15 μM, a value one order of magnitude bigger than the potency reported for auranofin. Compound 1 (5 mg kg−1 daily gavage for 14 days) caused a decrease in ex vivo spontaneous and ascorbate-induced lipid peroxidation in the homogenates of rat lung, heart muscle, spleen, liver, kidneys, testicles and brain as assessed by the thiobarbituric acid reactive substances. Importantly, in animals fed with 1, no discernible general toxicity was registered suggesting that this compound is well tolerated. Our results provide evidence for an efficient synthetic route to obtain gold containing anti-inflammatory drug candidates with balanced pro/anti-oxidative properties.
- This article is part of the themed collections: Metallomics Recent HOT articles and 6th International Symposium on Metallomics, Vienna, Austria


 Please wait while we load your content...
Please wait while we load your content...