The P-type ATPase inhibiting potential of polyoxotungstates†
Abstract
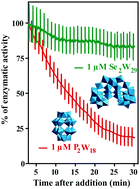
Polyoxometalates (POMs) are transition metal complexes that exhibit a broad diversity of structures and properties rendering them promising for biological purposes. POMs are able to inhibit a series of biologically important enzymes, including phosphatases, and thus are able to affect many biochemical processes. In the present study, we analyzed and compared the inhibitory effects of nine different polyoxotungstates (POTs) on two P-type ATPases, Ca2+-ATPase from skeletal muscle and Na+/K+-ATPase from basal membrane of skin epithelia. For Ca2+-ATPase inhibition, an in vitro study was performed and the strongest inhibitors were determined to be the large heteropolytungstate K9(C2H8N)5[H10Se2W29O103] (Se2W29) and the Dawson-type POT K6[α-P2W18O62] (P2W18) exhibiting IC50 values of 0.3 and 0.6 μM, respectively. Promising results were also shown for the Keggin-based POTs K6H2[CoW11TiO40] (CoW11Ti, IC50 = 4 μM) and Na10[α-SiW9O34] (SiW9, IC50 = 16 μM), K14[As2W19O67(H2O)] (As2W19, IC50 = 28 μM) and the lacunary Dawson K12[α-H2P2W12O48] (P2W12, IC50 = 11 μM), whereas low inhibitory potencies were observed for the isopolytungstate Na12[H4W22O74] (W22, IC50 = 68 μM) and the Anderson-type Na6[TeW6O24] (TeW6, IC50 = 200 μM). Regarding the inhibition of Na+/K+-ATPase activity, for the first time an ex vivo study was conducted using the opercular epithelium of killifish in order to investigate the effects of POTs on the epithelial chloride secretion. Interestingly, 1 μM of the most potent Ca2+-ATPase inhibitor, Se2W29, showed only a minor inhibitory effect (14% inhibition) on Na+/K+-ATPase activity, whereas almost total inhibition (99% inhibition) was achieved using P2W18. The remaining POTs exhibited similar inhibition rates on both ATPases. These results reveal the high potential of some POTs to act as P-type ATPase inhibitors, with Se2W29 showing high selectivity towards Ca2+-ATPase.
- This article is part of the themed collections: Metallomics 2018 Most Downloaded Articles and 6th International Symposium on Metallomics, Vienna, Austria


 Please wait while we load your content...
Please wait while we load your content...