A quantitative and temporal map of proteostasis during heat shock in Saccharomyces cerevisiae†
Abstract
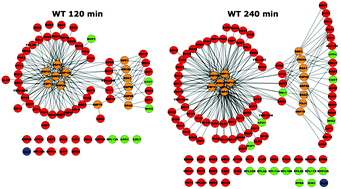
Temperature fluctuation is a common environmental stress that elicits a molecular response in order to maintain intracellular protein levels. Here, for the first time, we report a comprehensive temporal and quantitative study of the proteome during a 240 minute heat stress, using label-free mass spectrometry. We report temporal expression changes of the hallmark heat stress proteins, including many molecular chaperones, tightly coupled to their protein clients. A notable lag of 30 to 120 minutes was evident between transcriptome and proteome levels for differentially expressed genes. This targeted molecular response buffers the global proteome; fewer than 15% of proteins display significant abundance change. Additionally, a parallel study in a Hsp70 chaperone mutant (ssb1Δ) demonstrated a significantly attenuated response, at odds with the modest phenotypic effects that are observed on growth rate. We cast the global changes in temporal protein expression into protein interaction and functional networks, to afford a unique, time-resolved and quantitative description of the heat shock response in an important model organism.


 Please wait while we load your content...
Please wait while we load your content...