Sodium–glucose cotransporter 2 (SGLT-2) inhibitors: a new antidiabetic drug class
Abstract
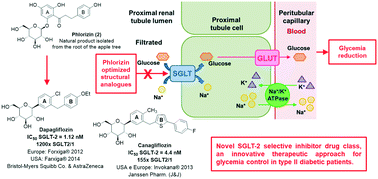
Diabetes mellitus is a chronic, complex and multifactorial disease associated characteristically with hyperglycemia. One of the most recently approved antidiabetic drug classes for clinical use are sodium–glucose cotransporter type 2 (SGLT-2) inhibitors. SGLT-2 is a protein expressed in the kidneys, responsible for glucose reabsorption from the glomerular filtrate to the plasma. It is known, nowadays, that diabetic patients show an increased glucose renal reabsorption capacity, caused by the overexpression of the SGLT-2 transporter, thus contributing to hyperglycemia. From establishing this correlation, the SGLT-2 transporter started to be considered as a therapeutic target of interest, culminating in the approval of the first antidiabetic SGLT-2 inhibitor, dapagliflozin (Forxiga® or Farxiga®, Bristol-Myers Squibb & AstraZeneca), in 2012 in Europe. On the other hand, canagliflozin (Invokana®, Janssen Pharmaceutical) was the first drug in this class to be approved by the FDA, the U.S. Food and Drug Administration, in 2013. This review concerns the discovery and development of the first representatives of this class of antidiabetic drugs, and the description of new optimized analogues that are currently in the clinical and preclinical stages of development.


 Please wait while we load your content...
Please wait while we load your content...