Mercaptoacetate thioesters and their hydrolysate mercaptoacetic acids jointly inhibit metallo-β-lactamase L1†
Abstract
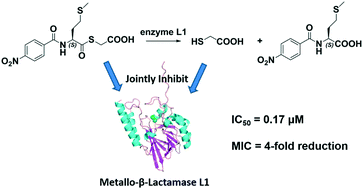
The ‘superbug’ infection caused by metallo-β-lactamases (MβLs) including L1 has grown into an emerging threat. To probe whether mercaptoacetate thioesters inhibiting L1 is a contribution of the thioester itself or its hydrolysate, ten mercaptoacetate thioesters 1–10 were synthesized, which specifically inhibited L1, exhibiting IC50 values ranging from 0.17 to 1.2 μM, and 8 was found to be the best inhibitor (IC50 = 0.17 μM). These thioesters restored the antimicrobial activity of cefazolin against E. coli expressing L1 by 2–4-fold. UV-vis monitoring showed that 1, 8 and 9 were unhydrolyzed in Tris buffer (pH 6.0–8.5), but hydrolyzed by L1; further HPLC monitoring indicated that 1/3 of the thioester 9 was converted to mercaptoacetic acid. STD-NMR monitoring suggested that both the thioester and its hydrolysate mercaptoacetic acid jointly inhibited L1.


 Please wait while we load your content...
Please wait while we load your content...