Triazole–diindolylmethane conjugates as new antitubercular agents: synthesis, bioevaluation, and molecular docking†
Abstract
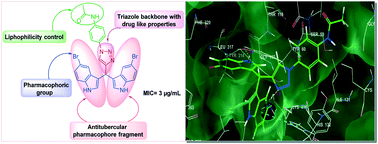
We describe the synthesis of novel triazole-incorporated diindolylmethanes (DIMs) using a molecular hybridization approach. The in vitro antitubercular activity of the DIMs against Mycobacterium tuberculosis H37Ra (ATCC 25177) was tested in the active and dormant state. Among all the synthesized conjugates, the compounds 6b, 6f, 6l, 6n, 6q, 6r, and 6s displayed good antitubercular activity against both the active and dormant Mtb H37Ra strain. The compound 6l exhibited good antitubercular activity against dormant Mtb H37Ra with an IC50 value of 1 μg mL−1 and IC90 (MIC) value of 3 μg mL−1. The compounds 6b, 6l, and 6r displayed good antitubercular activity against active Mtb H37Ra with IC50 values of 2.19, 1.52, and 0.22 μg mL−1, respectively. The compounds 6b, 6h, 6l, and 6s displayed more than 70% inhibition against the Gram-positive Bacillus subtilus strain at 3 μg mL−1. The molecular docking study showed the binding modes of the titled compounds in the active site of the DprE1 enzyme and assisted with elucidating a structural basis for the inhibition of Mycobacteria.


 Please wait while we load your content...
Please wait while we load your content...