Design, synthesis and biological evaluation of novel 1,3-diarylpyrazoles as cyclooxygenase inhibitors, antiplatelet and anticancer agents†
Abstract
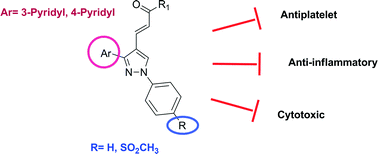
With the aim of achieving new compounds possessing both anti-inflammatory and antiplatelet activities, we synthesized (E)-3-[3-(pyridin-3/4-yl)-1-(phenyl/sulfonylmethylphenyl)-1H-pyrazol-4-yl]acrylamides, and evaluated their COX-1 and COX-2 inhibitory and antiplatelet activities. Since COX-2 inhibitory and antiplatelet compounds have anticancer potential, we also screened their antiproliferative effects against three human cancer cell lines. Compounds 5n, 5p, 5s, 10d, 10g and 10i were determined as dual COX-2 inhibitor/antiplatelet compounds. Compound 10h appeared to be a compound that exhibited antiplatelet activity without inhibiting the COX enzyme. Compounds 5h, 10a and 10i were the most effective derivatives which displayed antiproliferative activity against Huh7, MCF7 and HCT116 cells. Particularly, compound 10i, as the compound exhibiting the highest cytotoxic, antiplatelet and COX-2 inhibitory activity, was remarkable.


 Please wait while we load your content...
Please wait while we load your content...