Synthesis and evaluation of nuciferine and roemerine enantiomers as 5-HT2 and α1 receptor antagonists†
Abstract
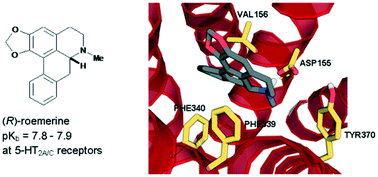
In this study, the (S)-enantiomers of the aporphine alkaloids, nuciferine and roemerine, were prepared via a synthetic route involving catalytic asymmetric hydrogenation and both stereoisomers were evaluated in vitro for functional activity at human 5-HT2 and adrenergic α1 receptor subtypes using a transforming growth factor-α shedding assay. Both enantiomers of each of the compounds were found to act as antagonists at 5-HT2 and α1 receptors. (R)-roemerine was the most potent compound at 5-HT2A and 5-HT2C receptors (pKb = 7.8–7.9) with good selectivity compared to (S)-roemerine at these two receptors and compared to its activity at 5-HT2B, α1A, α1B and α1D receptors.


 Please wait while we load your content...
Please wait while we load your content...