Structure-guided approach identifies a novel class of HIV-1 ribonuclease H inhibitors: binding mode insights through magnesium complexation and site-directed mutagenesis studies†
Abstract
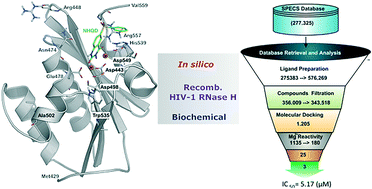
Persistent HIV infection requires lifelong treatment and among the 2.1 million new HIV infections that occur every year there is an increased rate of transmitted drug-resistant mutations. This fact requires a constant and timely effort in order to identify and develop new HIV inhibitors with innovative mechanisms. The HIV-1 reverse transcriptase (RT) associated ribonuclease H (RNase H) is the only viral encoded enzyme that still lacks an efficient inhibitor despite the fact that it is a well-validated target whose functional abrogation compromises viral infectivity. Identification of new drugs is a long and expensive process that can be speeded up by in silico methods. In the present study, a structure-guided screening is coupled with a similarity-based search on the Specs database to identify a new class of HIV-1 RNase H inhibitors. Out of the 45 compounds selected for experimental testing, 15 inhibited the RNase H function below 100 μM with three hits exhibiting IC50 values <10 μM. The most active compound, AA, inhibits HIV-1 RNase H with an IC50 of 5.1 μM and exhibits a Mg-independent mode of inhibition. Site-directed mutagenesis studies provide valuable insight into the binding mode of newly identified compounds; for instance, compound AA involves extensive interactions with a lipophilic pocket formed by Ala502, Lys503, and Trp (406, 426 and 535) and polar interactions with Arg557 and the highly conserved RNase H primer-grip residue Asn474. The structural insights obtained from this work provide the bases for further lead optimization.


 Please wait while we load your content...
Please wait while we load your content...