Membrane-active antimicrobial poly(amino-modified alkyl) β-cyclodextrins synthesized via click reactions†
Abstract
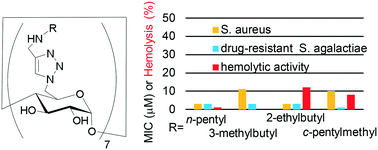
The emergence of drug-resistant bacteria has led to the high demand for new antibiotics. In this report, we investigated membrane-active antimicrobial β-cyclodextrins. These contain seven amino-modified alkyl groups on a molecule, which act as functional moieties to permeabilize bacterial cell membranes. The polyfunctionalization of cyclodextrins was achieved through a click reaction assisted by microwave irradiation. A survey using derivatives with systematically varied functionalities clarified the unique correlation of the antimicrobial activity of these compounds with their molecular structure and hydrophobicity/hydrophilicity balances. The optimum hydrophobicity for the compounds being membrane-active was specific to bacterial strains and animal cells; this led to specific compounds having selective toxicity against bacteria including multidrug-resistant pathogens. The results demonstrate that cyclodextrin is a versatile molecular scaffold for rationally designed structures and can be used for the development of new antibiotics.


 Please wait while we load your content...
Please wait while we load your content...