Design, synthesis and biological evaluation of 3′,4′,5′-trimethoxy flavonoid benzimidazole derivatives as potential anti-tumor agents†
Abstract
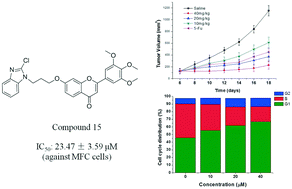
A series of 3′,4′,5′-trimethoxy flavonoids with benzimidazole linked by different chain alkanes have been designed and synthesized. The potential activity of these compounds as anti-tumor agents was evaluated by cytotoxicity assay in MGC-803 (human gastric cancer), MCF-7 (human breast cancer), HepG-2 (human hepatoma) and MFC (mouse gastric cancer) tumor cell lines. Among them, compound 15 7-(3-(2-chloro-1H-benzo[d]imidazol-1-yl)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one displayed the most potent antiproliferative activity, with IC50 values of 20.47 ± 2.07, 43.42 ± 3.56, 35.45 ± 2.03 μM and 23.47 ± 3.59 μM, respectively. The flow cytometry (FCM) results showed that compound 15 caused the cell cycle to be arrested in G1 phase and induced apoptosis of MFC cells in a dose-dependent manner. In addition, compound 15 exhibited a significant inhibitory effect on tumor growth in vivo. All the results outlined the great potential of compound 15 for further exploitation as anti-tumor agent.

 Please wait while we load your content...
Please wait while we load your content...