Novel valdecoxib derivatives by ruthenium(ii)-promoted 1,3-dipolar cycloaddition of nitrile oxides with alkynes – synthesis and COX-2 inhibition activity†
Abstract
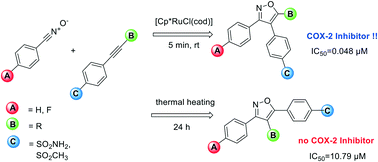
Novel valdecoxib-based cyclooxygenase-2 inhibitors were synthesized in one step via 1,3-dipolar cycloaddition of nitrile oxides with a series of eleven aryl alkynes, six of them described for the first time. Application of Ru(II)-catalysis leads preferably to the formation of the 3,4-diaryl-substituted isoxazoles, while under thermal heating with base the 3,5-diaryl substitution pattern is favoured. The new the 3,4-diaryl-substituted isoxazoles possessing a small substituent (H and Me) displayed high COX-2 inhibition affinity (IC50 = 0.042–0.073 μM) and excellent selectivity (COX-2 SI > 2000). In contrast, the 3,5-diaryl substituted compounds displayed almost no COX activity. The introduction of a 4-fluorophenyl substituent resulted in retained high COX-2 affinity, making these compounds together with the feasible one step reaction promising candidates for the development of fluorine-18 labelled radiotracers.


 Please wait while we load your content...
Please wait while we load your content...