Synthesis, characterization and biological application of 5-quinoline 1,3,5-trisubstituted pyrazole based platinum(ii) complexes†
Abstract
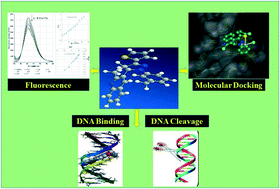
Square planar mononuclear platinum(II) complexes were synthesized in the presence of neutral bidentate heterocyclic (5-quinoline 1,3,5-tri-substituted pyrazole scaffold) ligands and K2PtCl4 salt. The synthesized compounds were characterized by micro-elemental analysis, FT-IR, UV-vis, 1H NMR, 13C NMR, TGA, mass spectrometry and molar conductivity. Their biological activities were investigated by in vitro brine shrimp lethality bioassay, in vitro antimicrobial study against five different pathogens, in vivo cellular level cytotoxicity against Schizosaccharomyces pombe cells, and in vitro anti-proliferation assay. The binding constant Ksv, Kb, Ka values of the complexes were determined by DNA interaction studies. The gel electrophoresis assay was carried out to examine the effect of the complexes on the DNA nuclease of pUC19 plasmid DNA. The docking energies of the ligands (L1–L5) and complexes (I–V) were observed in the range of −265.14 to −284.33 kJ mol−1. The synthesized Pt(II) complexes (I–V) were screened against the MCF-7 (human breast adenocarcinoma) and HCT-116 (human colon carcinoma) cancer cell lines.

 Please wait while we load your content...
Please wait while we load your content...