Synthesis and in vitro evaluation of substituted 3-cinnamoyl-4-hydroxy-pyran-2-one (CHP) in pursuit of new potential antituberculosis agents†
Abstract
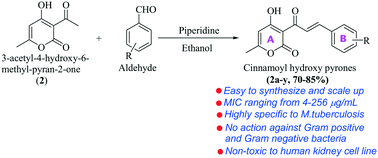
Tuberculosis is an ever-evolving infectious disease that urgently needs new drugs. In the search for new antituberculosis agents, a library of 3-cinnamoyl-4-hydroxy-6-methyl-2H-pyran-2-ones (CHPs) (2a–2y) was synthesized and evaluated against a standard virulent laboratory strain of Mycobacterium tuberculosis H37Rv. Out of 25 compounds, 11, 5, 7 and 2 (2a and 2u) showed least, moderate, good and appreciable activities, respectively, based on minimum inhibitory concentrations (MICs). Both 2a and 2u exhibited an MIC value of 4 μg ml−1, which was close to those of standard antituberculosis drugs ethambutol, streptomycin and levofloxacin. Neither 2a nor 2u showed any activity against Gram-positive or Gram-negative bacteria and even against non-tuberculous mycobacterium, i.e. Mycobacterium smegmatis. Thus, like the antituberculosis drugs rifampicin, isoniazid and pretomanid, they are highly TB specific. All the pyrone-based chalcones showed no recognizable level of cytotoxicity against normal human kidney cell line (HEK-293) up to 80 μM concentration and 11 exhibited an IC50 ≤ 100 μM (highest tested concentration). On further investigation, both 2a and 2u proved to be nontoxic against four human cell lines but 2a proved to be a better choice as it did not reach IC50 even at 100 μM (highest tested concentration) while the IC50 of 2u was around 80 μM. In conclusion, our results demonstrate that 2a is specific against M. tuberculosis with no appreciable toxicity; its activity matches that of some clinically approved antituberculosis drugs and it therefore merits further evaluation.


 Please wait while we load your content...
Please wait while we load your content...