Electrochemical sulfonylation of thiols with sulfonyl hydrazides: a metal- and oxidant-free protocol for the synthesis of thiosulfonates†
Abstract
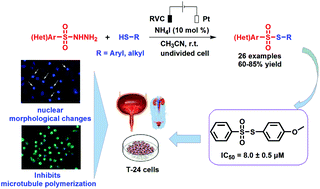
An efficient electrochemical transformation of structurally diverse sulfonyl hydrazides and thiols into thiosulfonates in the presence of ammonium iodide as redox catalyst and electrolyte in acetonitrile at ambient temperature is reported. Transition metal- and oxidant-free conditions are the striking features of this protocol. The in vitro cytotoxicity of all compounds is evaluated by MTT assay against four human cancer cell lines. The results reveal that 3ac and 3ag exhibit potential inhibitory activity against tumor cells. Furthermore, 3ag inhibits cell migration ability and tubulin polymerization in T-24 cells, leading to cell cycle arrest and apoptosis.


 Please wait while we load your content...
Please wait while we load your content...