Transition-metal-free oxidative cyclization of N-propargyl ynamides: stereospecific construction of linear polycyclic N-heterocycles†
Abstract
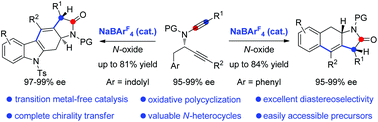
An efficient NaBArF4-catalyzed oxidative cascade cyclization of N-propargyl ynamides has been developed, where NaBArF4 is demonstrated for the first time to be a highly effective catalyst for promoting such an oxidative cyclization. Importantly, this transition metal-free process strongly supports that this oxidative catalysis proceeds by a Lewis acid-catalyzed SN2′ pathway, which is distinct from the related oxidative gold catalysis. This method leads to the efficient and practical synthesis of a diverse range of valuable polycyclic N-heterocycles with high diastereoselectivity and enantioselectivity by a chirality-transfer strategy.


 Please wait while we load your content...
Please wait while we load your content...