Anodic benzylic C(sp3)–H amination: unified access to pyrrolidines and piperidines†
Abstract
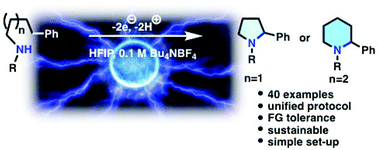
An electrochemical aliphatic C–H amination strategy was developed to access the important heterocyclic motifs of pyrrolidines and piperidines within a uniform reaction protocol. The mechanism of this unprecedented C–H amination strategy involves anodic C–H activation to generate a benzylic cation, which is efficiently trapped by a nitrogen nucleophile. The applicability of the process is demonstrated for 40 examples comprising both 5- and 6-membered ring formations.


 Please wait while we load your content...
Please wait while we load your content...