Supported gold- and silver-based catalysts for the selective aerobic oxidation of 5-(hydroxymethyl)furfural to 2,5-furandicarboxylic acid and 5-hydroxymethyl-2-furancarboxylic acid†
Abstract
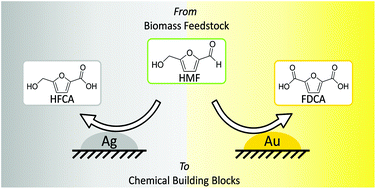
The sustainable synthesis of two important intermediates relevant for the production of bio-based polymers, 2,5-furandicarboxylic acid (FDCA) and 5-hydroxymethyl-2-furancarboxylic acid (HFCA), via oxidation of 5-(hydroxymethyl)furfural (HMF) was investigated using supported gold- and silver-based catalysts in water with air as the oxidant. High yields and selectivities for the production of FDCA (89%) and HFCA (≥98%) were achieved under the optimized reaction conditions with Au/ZrO2 and Ag/ZrO2 catalysts, respectively. While FDCA was mainly formed in the presence of gold catalysts at a maximum productivity of 67 molFDCA h−1 molAu−1, silver catalysts showed a remarkably high activity in aldehyde oxidation producing HFCA in almost quantitative yields with a maximum productivity of 400 molHFCA h−1 molAg−1. By variation of the reaction parameters, the Au/ZrO2 catalyst could be tuned to produce also HFCA, whereas the Ag/ZrO2 catalyst exclusively produced HFCA in a wide range of reaction parameters. The observed differences in catalyst selectivities can be taken as a starting point for further mechanistic investigation on the oxidation of HMF, contributing to a fundamental understanding of this reaction which is particularly important for establishing the production of bio-based polymers.
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...