The CO2 capturing ability of cellulose dissolved in NaOH(aq) at low temperature†
Abstract
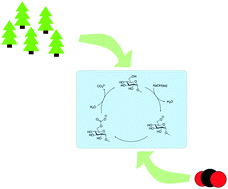
Herein, we explore the intrinsic ability of cellulose dissolved in NaOH(aq) to reversibly capture CO2. The stability of cellulose solutions differed significantly when adding CO2 prior to or after the dissolution of cellulose. ATR-IR spectroscopy on cellulose regenerated from the solutions, using ethanol, revealed the formation of a new carbonate species likely to be cellulose carbonate. To elucidate the interaction of cellulose with CO2 at the molecular level, a 13C NMR spectrum was recorded on methyl α-D-glucopyranoside (MeO-Glcp), a model compound, dissolved in NaOH(aq), which showed a difference in chemical shift when CO2 was added prior to or after the dissolution of MeO-Glcp, without a change in pH. The uptake of CO2 was found to be more than twice as high when CO2 was added to a solution after the dissolution of MeO-Glcp. Altogether, a mechanism for the observed CO2 capture is proposed, involving the formation of an intermediate cellulose carbonate upon the reaction of a cellulose alkoxide with CO2. The intermediate was observed as a captured carbonate structure only in regenerated samples, while its corresponding NMR peak in solution was absent. The reason for this is plausibly a rather fast hydrolysis of the carbonate intermediate by water, leading to the formation of CO32−, and thus increased capture of CO2. The potential of using carbohydrates as CO2 capturing agents in NaOH(aq) is shown to be simple and resource-effective in terms of the capture and regeneration of CO2.


 Please wait while we load your content...
Please wait while we load your content...